Chapter 1. Impact 19.1
Impact…ON BIOCHEMISTRY: I19.1 Ion channels
Controlled transport of molecules and ions across biological membranes is at the heart of a number of key cellular processes, such as the transmission of nerve impulses, the transfer of glucose into red blood cells, and the synthesis of ATP by oxidative phosphorylation (Impact I6.1). Here we examine some of the ways in which ions cross the alien environment of the lipid bilayer.
The thermodynamic tendency to transport an ion through the membrane is partially determined by a concentration gradient (more precisely, an activity gradient) across the membrane, which results in a difference in molar Gibbs energy between the inside and the outside of the cell, and a transmembrane potential gradient, which is due to the different potential energy of the ions on each side of the bilayer. There is a tendency, called passive transport, for a species to move spontaneously down concentration and membrane potential gradients. It is also possible to move a species against these gradients, but now the flow is not spontaneous and must be driven by an exergonic process, such as the hydrolysis of ATP. This process is called active transport.
The transport of ions into or out of a cell needs to be mediated (that is, facilitated by other species) because the hydrophobic environment of the membrane is inhospitable to ions. There are two mechanisms for ion transport: mediation by a carrier molecule and transport through a ‘channel former’, a protein that creates a hydrophilic pore through which the ion can pass. An example of a channel former is the polypeptide gramicidin A, which increases the membrane permeability to cations such as H+, K+, and Na+.
Ion channels are proteins that effect the movement of specific ions down a membrane potential gradient. They are highly selective, so there is a channel protein for Ca2+, another for Cl-, and so on. The opening of the gate may be triggered by potential differences between the two sides of the membrane or by the binding of an ‘effectormolecule’ to a specific receptor site on the channel.
Ions such as H+, Na+, K+, and Ca2+ are often transported actively across membranes by integral proteins called ion pumps. Ion pumps are molecular machines that work by adopting conformations that are permeable to one ion but not others depending on the state of phosphorylation of the protein. Because protein phosphorylation requires dephosphorylation of ATP, the conformational change that opens or closes the pump is endergonic and requires the use of energy stored during metabolism.
The structures of a number of channel proteins have been obtained by the now traditional X-ray diffraction techniques described in Topic 18A. Information about the flow of ions across channels and pumps is supplied by the patch clamp technique. One of many possible experimental arrangements is shown in Fig. I19.1. With mild suction, a ‘patch’ of membrane from a whole cell or a small section of a broken cell can be attached tightly to the tip of a micropipette filled with an electrolyte solution and containing an electronic conductor, the so-called ‘patch electrode’. A potential difference (the ‘clamp’) is applied between the patch electrode and an intracellular electronic conductor in contact with the cytosol of the cell. If the membrane is permeable to ions at the applied potential difference, a current flows through the completed circuit. Using narrow micropipette tips with diameters of less than 1 μm, ion currents of a few picoamperes (1 pA = 10-12 A) have been measured across sections of membranes containing only one ion channel protein.
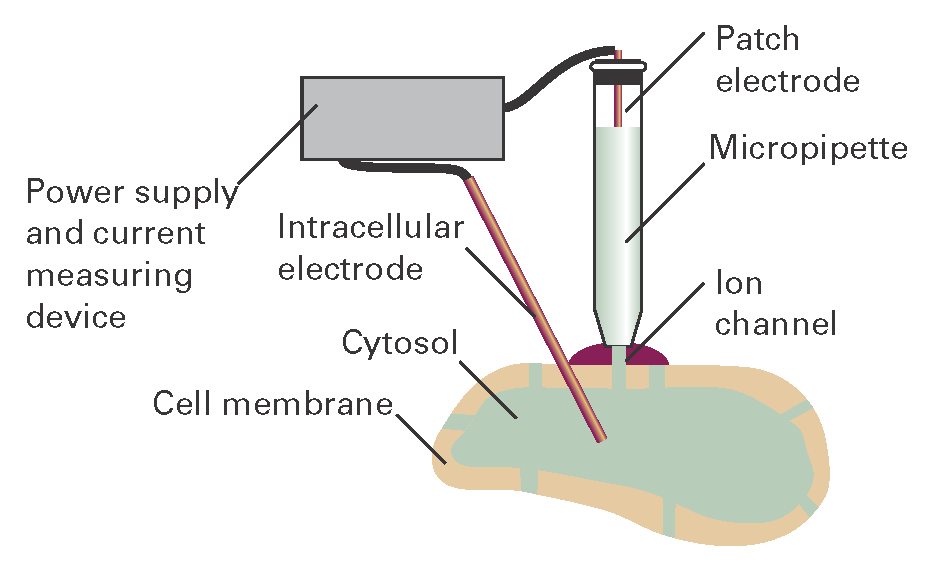
Figure I19.1 A representation of the patch clamp technique for the measurement of ionic currents through membranes in intact cells. A section of membrane containing an ion channel is in tight contact with the tip of a micropipette containing an electrolyte solution and the patch electrode.
A detailed picture of the mechanism of action of ion channels has emerged from analysis of patch clamp data and structural data. Here we focus on the K+ ion channel protein, which, like all other mediators of ion transport, spans the membrane bilayer (Fig. I19.2). The pore through which ions move has a length of 3.4 nm and is divided into two regions: a wide region with a length of 2.2 nm and diameter of 1.0 nm and a narrow region with a length of 1.2 nm and diameter of 0.3 nm. The narrow region is called the ‘selectivity filter’ of the K+ ion channel because it allows only K+ ions to pass.
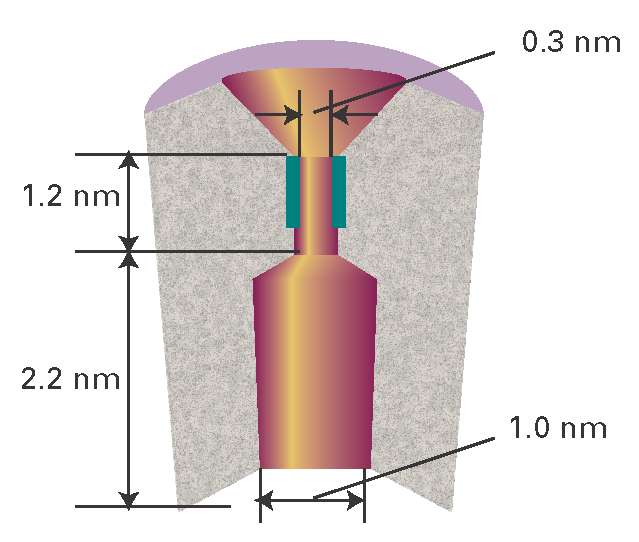
Figure I19.2 A schematic representation of the cross-section of a membrane-spanning K+ ion channel protein. The pore through which ions move is divided into two regions: a wide region and a narrow region, the selectivity filter. The selectivity filter has a number of carbonyl groups (shown in dark green) that grip K+ ions. As explained in the text, electrostatic repulsions between two bound K+ ions ‘encourage’ ionic movement through the selectivity filter and across the membrane.
Filtering is a subtle process that depends on ionic size and the thermodynamic tendency of an ion to lose its hydrating water molecules. Upon entering the selectivity filter, the K+ ion is stripped of its hydrating shell and is then gripped by carbonyl groups of the protein. Dehydration of the K+ ion is endergonic (\(\Delta\)dehydG\(\oplus\) = +203 kJ mol–1), but is driven by the energy of interaction between the ion and the protein. The Na+ ion, though smaller than the K+ ion, does not pass through the selectivity filter of the K+ ion channel because interactions with the protein are not sufficient to compensate for the high Gibbs energy of dehydration of Na+ (\(\Delta\)dehydG\(\oplus\) = +301 kJ mol–1). More specifically, a dehydrated Na+ ion is too small and cannot be held tightly by the protein carbonyl groups, which are positioned for ideal interactions with the larger K+ ion. In its hydrated form, the Na+ ion is too large (larger than a dehydrated K+ ion), does not fit in the selectivity filter, and does not cross the membrane.
Though very selective, a K+ ion channel can still let other ions pass through. For example, K+ and Tl+ ions have similar radii and Gibbs energies of dehydration, so Tl+ can cross the membrane. As a result, Tl+ is a neurotoxin because it replaces K+ in many neuronal functions.
The efficiency of transfer of K+ ions through the channel can also be explained by structural features of the protein. For efficient transport to occur, a K+ ion must enter the protein, but then must not be allowed to remain inside for very long so that, as one K+ ion enters the channel from one side, another K+ ion leaves from the opposite side. An ion is lured into the channel by water molecules about halfway through the length of the membrane. Consequently, the thermodynamic cost of moving an ion from an aqueous environment to the less hydrophilic interior of the protein is minimized. The ion is ‘encouraged’ to leave the protein by electrostatic interactions in the selectivity filter, which can bind two K+ ions simultaneously, usually with a bridging water molecule. Electrostatic repulsion prevents the ions from binding too tightly, minimizing the residence time of an ion in the selectivity filter, and maximizing the transport rate.