Chapter 1. Impact 6.2
Impact…ON CHEMICAL ANALYSIS: I6.2 Species-selective electrodes
An ion-selective electrode, one example of a ‘species-selective’ electrode, is an electrode that generates a potential in response to the presence of a solution of specific ions. An example is the glass electrode (Fig. I6.1), which is sensitive to hydrogen ion activity, and has a potential proportional to pH. It is filled with a phosphate buffer containing Cl– ions, and conveniently has E = 0 when the external medium is at pH = 6. It is necessary to calibrate the glass electrode before use with solutions of known pH.
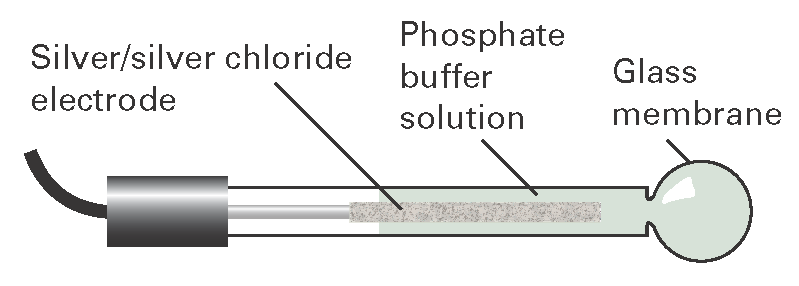
Fig. I6.1 The glass electrode. It is commonly used in conjunction with a calomel electrode that makes contact with the test solution through a salt bridge.
The responsiveness of a glass electrode to the hydrogen ion activity is a result of complex processes at the interface between the glass membrane and the solutions on either side of it. The membrane itself is permeable to Na+ and Li+ ions but not to H+ ions. Therefore, the potential difference across the glass membrane must arise by a mechanism different from that responsible for biological transmembrane potentials. A clue to the mechanism comes from a detailed inspection of the glass membrane, for each face is coated with a thin layer of hydrated silica (Fig. I6.2). The hydrogen ions in the test solution modify this layer to an extent that depends on their activity in the solution, and the charge modification of the outside layer is transmitted to the inner layer by the Na+ and Li+ ions in the glass. The hydrogen ion activity gives rise to a membrane potential by this indirect mechanism.
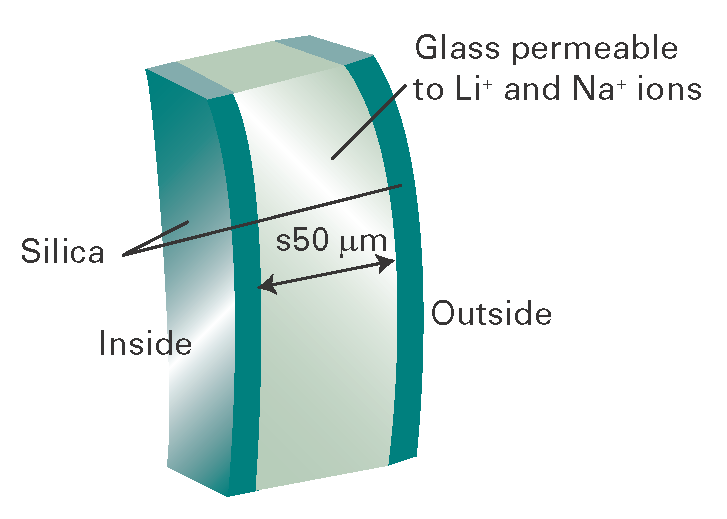
Fig. I6.2 A section through the wall of a glass electrode.
Electrodes sensitive to hydrogen ions, and hence to pH, are typically glasses based on lithium silicate doped with heavy-metal oxides. The glass can also be made responsive to Na+, K+, and ions by being doped with Al2O3 and B2O3.
A suitably adapted glass electrode can be used to detect the presence of certain gases. A simple form of a gas-sensing electrode consists of a glass electrode contained in an outer sleeve filled with an aqueous solution and separated from the test solution by a membrane that is permeable to gas. When a gas such as sulfur dioxide or ammonia diffuses into the aqueous solution, it modifies its pH, which in turn affects the potential of the glass electrode. The presence of an enzyme that converts a compound, such as urea or an amino acid, into ammonia, which then affects the pH, can be used to detect these organic compounds.
Somewhat more sophisticated devices are used as ion-selective electrodes that give potentials according to the presence of specific ions present in a test solution. In one arrangement, a porous lipophilic (hydrocarbon-attracting) membrane is attached to a small reservoir of a hydrophobic (water-repelling) liquid, such as dioctylphenylphosphonate, that saturates it (Fig. I6.3). The liquid contains an agent, such as (RO)2PO2–, with R a C8 to C18 chain, that acts as a kind of solubilizing agent for the ions with which it can form a complex. The complex’s ions are able to migrate through the lipophilic membrane, and hence give rise to a transmembrane potential, which is detected by a silver/silver chloride electrode in the interior of the assembly. Electrodes of this construction can be designed to be sensitive to a variety of ionic species, including calcium, zinc, iron, lead, and copper ions.
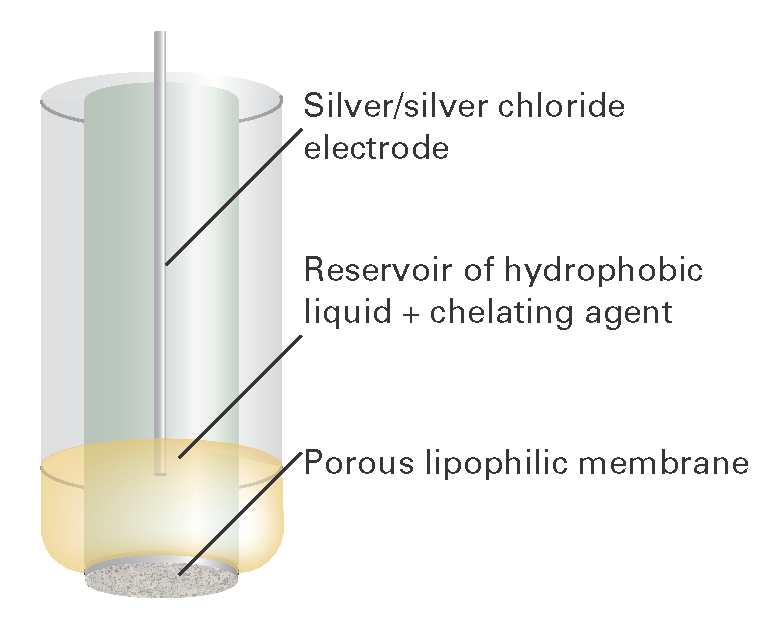
Fig. I6.3 The structure of an ion-selective electrode. Chelated ions are able to migrate through the lipophilic membrane.
In theory, the transmembrane potential should be determined entirely by differences in the activity of the species that the electrode was designed to detect. In practice, a small potential difference, called the asymmetry potential, is observed even when the activity of the test species is the same on both sides of the membrane. The asymmetry potential is due to the fact that it is not possible to manufacture a membrane material that has the same structure and the same chemical properties throughout. Furthermore, all species-selective electrodes are sensitive to more than one species. For example, a Na+ selective electrode also responds, albeit less effectively, to the activity of K+ ions in the test solution. As a result of these effects, the potential of an electrode sensitive to species X+ that is also susceptible to interference by species Y+ is given by a modified form of the Nernst equation:
\( E_{cell} = E_{ap} + \frac{{\beta}RT}{F} {ln(a_{X^+} + k_{X,Y}a_{Y^+})}\)
where Eap is the asymmetry potential, β is an experimental parameter that captures deviations from the Nernst equation, and kX,Y is the selectivity coefficient of the electrode and is related to the response of the electrode to the interfering species Y+. A value of β = 1 indicates that the electrode responds to the activity of ions in solution in a way that is consistent with the Nernst equation and, in practice, most species-selective electrodes of high quality have β ≈ 1. The selectivity coefficient, and hence interference effects, can be minimized when designing and manufacturing a species-selective electrode. For precise work, it is necessary to calibrate the response of the electrode by measuring Eap, β, and kX,Y before performing experiments on solutions of unknown concentration of X+.