Chapter 1. Impact 14.2
Impact …ON MATERIALS SCIENCE: I14.2 Liquid crystals
A mesophase is a phase intermediate between solid and liquid. Mesophases are of great importance in biology, for they occur as lipid bilayers and in vesicular systems. A mesophase may arise when molecules have highly non-spherical shapes, such as being long and thin (1), or disk-like (2). When the solid melts, some aspects of the long-range order characteristic of the solid may be retained, and the new phase may be a liquid crystal, a substance having liquid-like imperfect long-range order in at least one direction in space but positional or orientational order in at least one other direction. Calamitic liquid crystals (from the Greek word for reed) are made from long and thin molecules, whereas discotic liquid crystals are made from disk-like molecules. A thermotropic liquid crystal displays a transition to the liquid crystalline phase as the temperature is changed. A lyotropic liquid crystal is a solution that undergoes a transition to the liquid crystalline phase as the composition is changed.
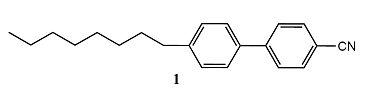
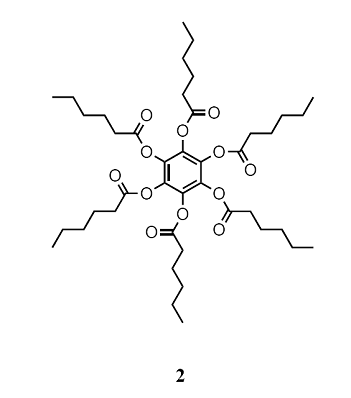
One type of retained long-range order gives rise to a smectic phase (from the Greek word for soapy), in which the molecules align themselves in layers (Fig. 1). Other materials, and some smectic liquid crystals at higher temperatures, lack the layered structure but retain a parallel alignment; this mesophase is called a nematic phase (from the Greek for thread, which refers to the observed defect structure of the phase). In the cholesteric phase (from the Greek for bile solid) the molecules lie in sheets at angles that change slightly between each sheet. That is, they form helical structures with a pitch that depends on the temperature. As a result, cholesteric liquid crystals diffract light and have colours that depend on the temperature. Disk-like molecules such as (2) can form nematic and columnar mesophases. In the latter, the aromatic rings stack one on top of the other and are separated by very small distances (less than 0.5 nm). Figure 2 shows the pressure–temperature phase diagram of octylcyanobiphenyl, which is widely used in liquid crystal displays.
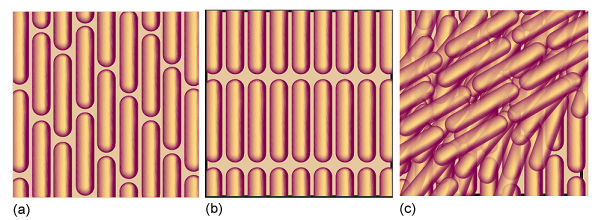
Fig. 1 The arrangement of molecules in (a) the nematic phase, (b) the smectic phase, and (c) the cholesteric phase of liquid crystals. In the cholesteric phase, the stacking of layers continues to give a helical arrangement of molecules.
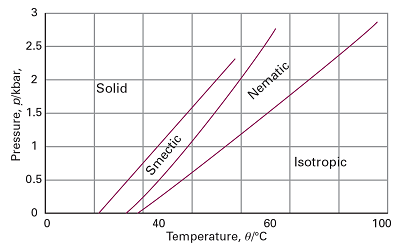
Fig. 2 The pressure–temperature diagram of octylcyanobiphenyl (8CB). (Based on R. Shashidhar and G. Venkatesh, J. de Physique Colloque, 40, C3 (1979).)
The optical properties of nematic liquid crystals are anisotropic, meaning that they depend on the relative orientation of the molecular assemblies with respect to the polarization of the incident beam of light. Nematic liquid crystals also respond in special ways to electric fields. Together, these unique optical and electrical properties form the basis of operation of liquid crystal displays (LCDs). In a ‘twisted nematic’ LCD, the liquid crystal is held between two flat plates about 10 mm apart. The inner surface of each plate is coated with a transparent conducting material, such as indium–tin oxide. The plates also have a surface that causes the liquid crystal to adopt a particular orientation at its interface and are typically set at 90° to each other but 270° in a ‘supertwist’ arrangement. The entire assembly is set between two polarizers, optical filters that allow light of only one specific plane of polarization to pass. The incident light passes through the outer polarizer, then its plane of polarization is rotated as it passes through the twisted nematic and, depending on the setting of the second polarizer, will pass through (if that is how the second polarizer is arranged). When a potential difference is applied across the cell, the helical arrangement is lost and the plane of the light is no longer rotated and will be blocked by the second polarizer.
Although there are many liquid crystalline materials, some difficulty is often experienced in achieving a technologically useful temperature range for the existence of the mesophase. To overcome this difficulty, mixtures can be used. An example of the type of phase diagram that is then obtained is shown in Fig. 3. As can be seen, the mesophase exists over a wider range of temperatures than either liquid crystalline material alone.
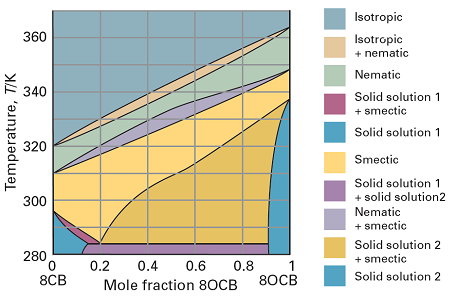
Fig. 3 The phase diagram at 1 atm for a binary system of two liquid crystalline materials, octylcyanobiphenyl (8CB) and octyloxycyanobiphenyl (8OCB). (Based on P. Rushikesh, A. Matkar, and T. Kyua, J. Chem. Phys., 124, 224902 (2006).)